Particle Size Analysis of Salt-Formed Active Pharmaceutical Ingredients
2024-10-21Application Note
This study measures the particle size of salt-formed active pharmaceutical ingredients (APIs) using the Bettersizer 2600 with BT-80N Pro. Given the high water solubility of these APIs, organic solvents were utilized for dispersion. Three salt-formed APIs were analyzed, demonstrating good repeatability and unique particle size characteristics. The instrument effectively aids in optimizing drug formulation and manufacturing processes.
| Product | Bettersizer 2600 |
| Industry | Pharmaceuticals |
| Sample | Salt-formed active pharmaceutical ingredients (APIs) |
| Measurement Type | Particle Size |
| Measurement Technology | Laser Diffraction |
Jump to a section:
Introduction
In the pharmaceutical industry, the particle size of active pharmaceutical ingredients (APIs) plays a critical role in the development and quality assurance of drug products. Particle size can significantly influence key performance factors such as dissolution rate, bioavailability, content uniformity, and the stability of the final drug product. Additionally, particle size affects the manufacturability of the product by impacting flowability, blending uniformity, and compatibility, factors that ultimately contribute to the safety, effectiveness, and overall quality of the drug product.
The Biopharmaceutics Classification System (BCS) categorizes APIs into four categories (I, II, III, IV) based on their solubility and permeability characteristics. Class II drugs, which have low solubility but high permeability, are particularly challenging because their poor solubility can limit the drug dissolution, potentially reducing bioavailability and therapeutic efficacy. To overcome this limitation, pharmaceutical researchers often employ salt formation strategies. A “salt-formed API” refers to an API that has been chemically modified by forming a salt with another compound, typically an acid or base, to improve its solubility physicochemical properties to enhance the bioavailability of the compound.
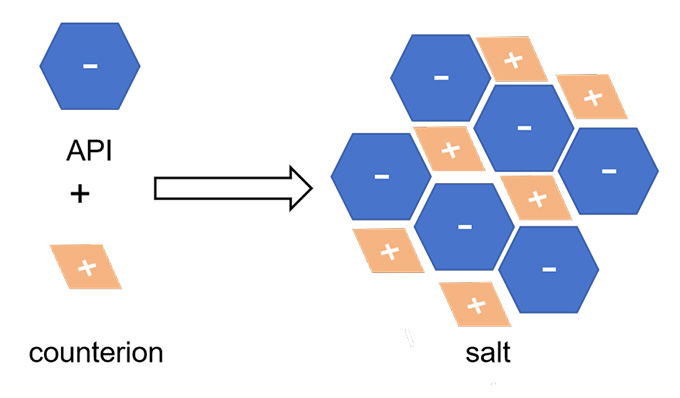
Figure 1: Salt formation
Given the importance of salt-formed APIs, accurate particle size analysis is essential. The laser diffraction (LD) technique is widely used for particle size analysis, particularly the wet dispersion method. However, this method encounters challenges when applied to highly water-soluble salt-formed APIs. The traditional wet dispersion medium, water, is no longer suitable as it can cause rapid dissolution of these compounds, compromising the accuracy of the measurement. To address this, organic solvents are often used as an alternative dispersion medium. While effective, organic solvents introduce additional complexities, such as stricter material compatibility requirements for the instrument, and more intricate dispersion processes due to the differences in viscosity and density. These facts can affect the accuracy and reliability of particle size measurements.
In this application note, the Bettersizer 2600, equipped with the BT-80N Pro accessory, was used to measure the particle size of different salt-formed APIs dispersed in organic solvents.
Experimental
Materials
Three different salt-formed active pharmaceutical ingredients (APIs) were selected for particle size analysis: sulfate (#1), sodium salt (#2) and hydrochloride (#3).
Methods
The particle size distribution of three samples was measured using the Bettersizer 2600 equipped with the BT-80N Pro, an anti-corrosive dispersion unit.
The BT-80N Pro is specially designed with unique features, including:
- Ability to withstand organic and corrosive solvents.
- Operate under the control of the Bettersizer software, which reduces human error and ensures reproducible results.

Bettersizer 2600 + BT-80N Pro
To improve data accuracy, a pre-dispersion step was performed prior to particle size analysis. The sample pre-dispersion guide for wet dispersion was shown in Figure 2. The sample suspensions were prepared by mixing 40 mg of each dry powder with 40 mL of white oil (also known as liquid paraffin), followed by stirring. The suspensions were then subjected to ultrasonic treatment for 3 minutes using an ultrasonic probe to ensure stable dispersion.
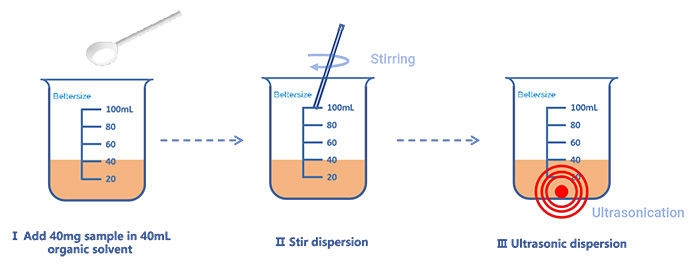
Figure 2: Sample pre-dispersion guide for wet dispersion
Once the pre-dispersion was completed, the suspensions were transferred in aliquots into the circulation tank of BT-80N Pro until an appropriate obscuration level was achieved. The measurements were conducted at a circulation speed of 800 rpm.
Each sample was tested six times to assess result repeatability and the relative standard deviation was calculated to assess the consistency of the results.
Results
Using Sample #3 as an example, the results are presented in Figure 3. Over a 6-minuted dispersion process, the D10, D50, and D90 values of Sample #3 exhibited minimal variation. This indicates that the sample disperses uniformly in the organic solvent and maintains stability, facilitating the acquisition of reliable data.
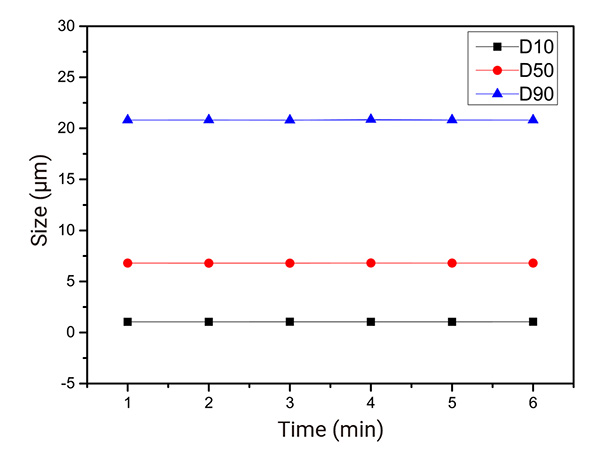
Figure 3: Changes in D10, D50, and D90 of Sample #3 over 6 minutes of dispersion.
As shown in Table 1, the relative standard deviations (RSD) for the D10, D50, and D90 values of all three samples across six tests were consistently below 0.5%. This high level of repeatability confirms that the samples were adequately dispersed, ensuring both the reliability of the method and the accuracy of particle size distribution (PSD) results.
Table 1: Repeatability of the results across six tests for each sample.
|
|
%RSD D10 |
%RSD D50 |
%RSD D90 |
|
#1 |
0.37 |
0.44 |
0.38 |
|
#2 |
0.10 |
0.22 |
0.14 |
|
#3 |
0.11 |
0.07 |
0.10 |
As shown below, Figure 4 illustrates the particle size distribution (PSD) of the three salt-formed APIs, while Table 2, summarizes their D10, D50 and D90 values. Each salt-formed APIs exhibits a distinct particle size profile, which can be attributed to differences in their crystal structure and production processes. Variations in crystal growth patterns, influences by crystal structure as well as factors such as crystallization conditions, drying methods, and milling techniques can lead to significant differences in particle size.
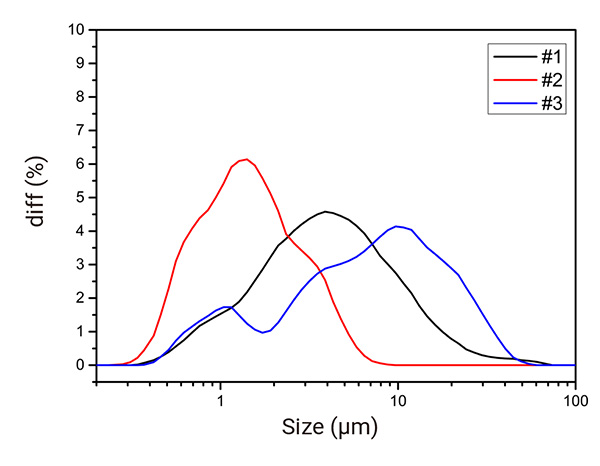
Figure 4: Particle size distribution of the three salt-formed APIs.
Table 2: D10, D50 and D90 values of the three salt-formed APIs.
|
|
D10 (μm) |
D50 (μm) |
D90 (μm) |
|
#1 |
1.122 |
3.716 |
11.49 |
|
#2 |
0.588 |
1.319 |
3.127 |
|
#3 |
1.048 |
6.797 |
20.82 |
Conclusion
The Bettersizer 2600 with its modular design, offers exceptional versatility for particle size analysis. Its diverse dispersion system modules support both dry and wet dispersion methods, allowing it to meet a wide range of testing requirements.
In this application note, the Bettersizer 2600 paired with the BT-80N Pro accessory was selected to accommodate the specific characteristics of salt-formed APIs. The relative standard deviations (RSD) of the D50 values are 0.44%, 0.22%, and 0.07%, demonstrating excellent repeatability in the measurement. With its high accuracy and stable results, the Bettersizer 2600 proves to be a reliable and advanced instrument for analyzing the particle size of salt-formed drugs. The capability supports improvements in drug formulation, ensures product consistency, and helps optimize manufacturing processes.
About the Author
 |
Zhichao Han Application Engineer @ Bettersize Instruments |
|
Bettersizer 2600 Laser Diffraction Particle Size Analyzer |
|
|
|
|
Rate this article

