Assessing the Cleaning Efficacy of Detergents with BeScan Lab
2024-07-30Application Note
The BeScan Lab system offers rapid and precise assessments of emulsion stability, aiding in evaluating the cleaning effectiveness of detergents. By streamlining the R&D process and ensuring consistent product quality, the BeScan Lab system effectively supports the development of high-performance detergent formulations.
| Product | BeScan Lab |
| Industry | Chemicals |
| Sample | Detergent |
| Measurement Type | Stability |
| Measurement Technology | Static Multiple Light Scattering (SMLS) |
Jump to a section:
Introduction
Surfactants, with their amphiphilic molecular structure, are crucial in cleaning and emulsification due to their ability to adsorb at water and other media interfaces. In cleaning products, they are key for effective dirt removal. Surfactants work by lowering water's surface tension, enhancing its wetting ability on surfaces, and preventing dirt from redepositing. They also modify the wetting properties of solid contaminants and use charge repulsion to aid in dirt removal.
The effectiveness of cleaning depends on factors like mechanical force and temperature, which influence the detergent's emulsifying ability and the stability of the emulsion. Emulsion stability is critical for dispersing and suspending dirt.
This study explores the efficiency of different detergent formulations in removing soybean oil at various temperatures. Using the BeScan Lab stability analyzer, we accurately measured emulsion stability, aiming to develop a quick and precise method to evaluate the emulsifying capacity of detergents, aiding researchers in formulating and optimizing products.
Principle
BeScan Lab utilizes Static Multiple Light Scattering (SMLS) technology, directing 850 nm light pulses vertically into the sample. Data is collected at 20 µm intervals, monitoring fluctuations in the backscattering and transmission levels over time to detect sample instability.
Governed by the Mie theory, signals of transmitted and backscattered light directly correlate with particle concentration (φ) and size (d). BeScan Lab provides an Instability Index (IUS) to assess the stability of dispersions, calculated by summing signal variations across sample height and time. A higher IUS indicates lower stability, automatically determined for each scan using a specific formula:
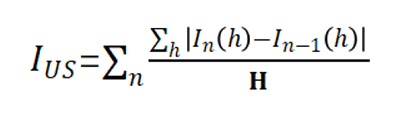
Experimental
a) Zeta Potential and Particle Size Measurement
The BeNano 180 Zeta Pro was used to measure the zeta potential and particle size of two detergent emulsions and emulsified samples with 0.625% soybean oil. Each sample was measured in triplicate, and average results were calculated.
b) Emulsion Stability Measurement
For each 15 ml detergent-oil-water emulsion, ultrasonic emulsification was performed, and the samples were placed in BeScan Lab’s sample cells. Scanning was conducted every minute for 60 minutes at 45 °C and 30 °C, separately.
 |
 |
Figure 1. BeScan Lab and BeNano 180 Zeta Pro
Results
Zeta Potential Results
The high cleaning efficiency of detergents is largely attributed to the surfactants they contain. These molecules, when reaching a certain concentration (critical micelle concentration, CMC), form micelles that aid in dissolving and dispersing grease in water, thereby creating stable emulsions.
Table 1. Zeta Potential Results
| Sample | Mean Zeta Potential (mV) |
| #1 detergent-water | -52.73 |
| #2 detergent-water | -36.37 |
| #1 detergent-oil-water | -73.42 |
| #2 detergent-oil-water | -76.72 |
Experimental results indicate that both detergents contain anionic surfactants, as evidenced by their negative zeta potential values. A higher zeta potential value reflects stronger electrostatic repulsion between droplets and greater stability. Detergent #1 has a zeta potential of -52.73 mV, while detergent #2 has a lower value of -36.37 mV, indicating that detergent #1 has stronger charge and stability.
Upon adding soybean oil, the absolute value of the zeta potential for both detergent systems increased further. Detergent #1 reached -73.42 mV, and detergent #2 reached -76.72 mV. This increase in zeta potential suggests that both detergents form stable emulsions in the initial stages, with the electrostatic repulsion between droplets helping to maintain a uniform dispersion.
Particle Size Results
The size of oil droplets in an emulsion is critical for their stability and dispersion. Ultrasonic emulsification effectively uses cavitation to apply intense temperatures and pressures, reducing droplets to a fine 300-400 nanometer scale and creating a consistently dispersed emulsion.
Table 2. Particle Size Results
| Sample | Z-ave(nm) |
| # 1 detergent-oil-water(0 min) | 330.32 |
| #1 detergent-oil-water(2 hr) | 336.54 |
| #2 detergent-oil-water(0 min) | 342.51 |
| #2 detergent-oil-water(2 hr) | 346.14 |
Table 2 indicates that #1 produces emulsions with smaller particle sizes compared to #2, likely due to its superior surfactants, which reduce interfacial tension and enhance oil dispersion. The stable particle size of the emulsions after two hours suggests excellent stability.
Stability Results
1) Delta Backscattered Signal at 30 °C
Figure 2 illustrates changes in delta backscattered signals for emulsions of two detergents at 30 °C and 45 °C, plotted against sample height over time.
In the one-hour measurement, the backscattered signals revealed trends for both emulsions (as shown in Figure 2):
At 30 °C, the top layer showed increased signals due to oil droplets rising, while the middle and bottom layers exhibited decreased signals, suggesting initial good dispersion followed by a decline in stability as oil droplets ascended over time.
At 45 °C, similar trends were observed with increased top layer signals and decreased middle and bottom layer signals, indicating the formation of larger droplets that rose, thus reducing the concentration in lower layers.
In conclusion, phase separation occurred in both emulsions, forming an oil phase, with more pronounced changes observed at the higher temperature of 45 °C compared to 30 °C.
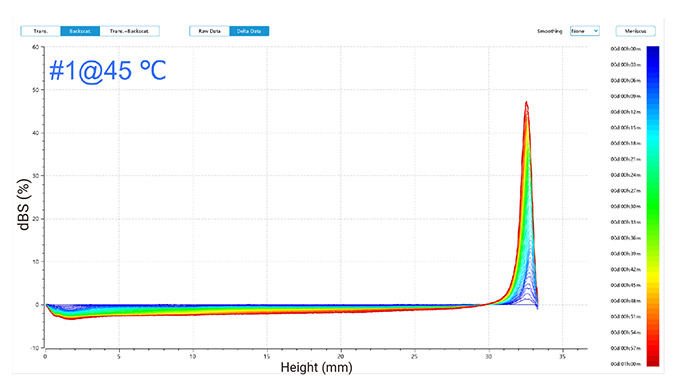
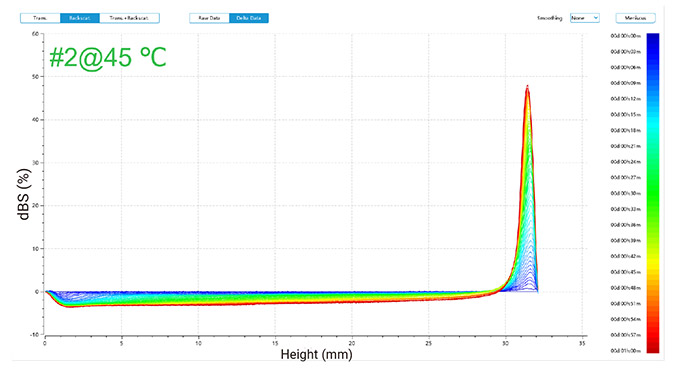
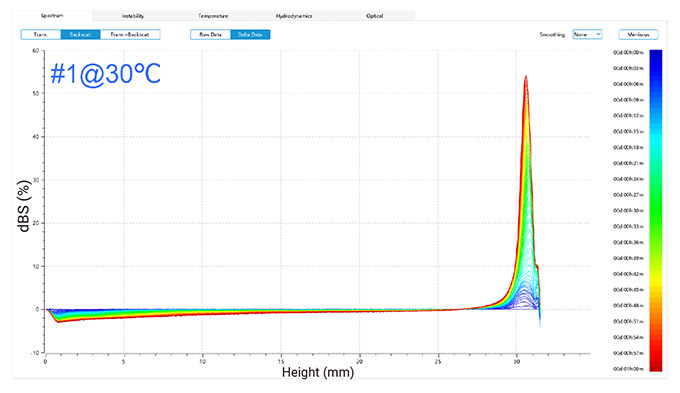
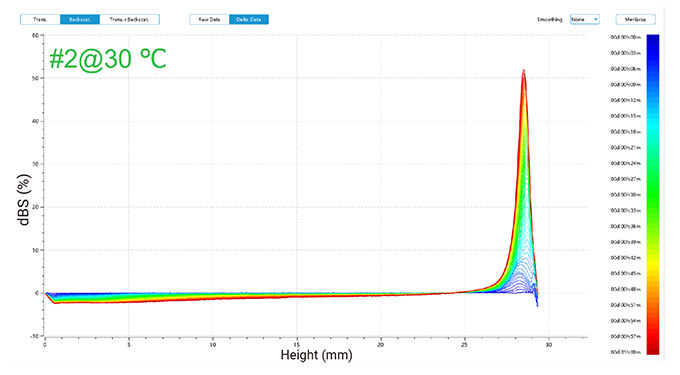
Figure 2. Delta backscattered signals of two detergent emulsions at 30 °C and 45 °C
2) Global Instability Index
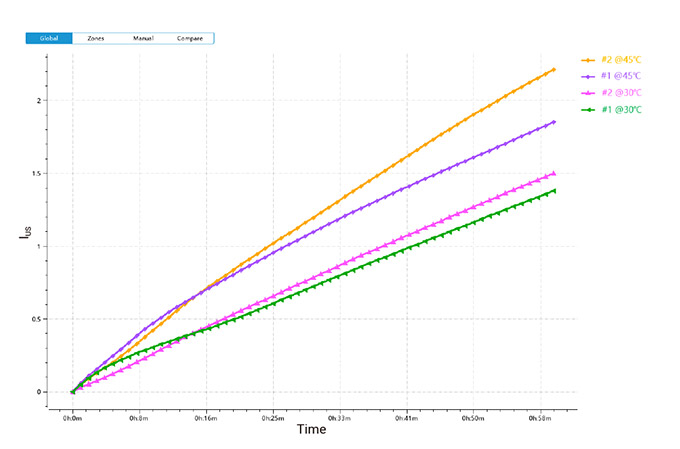
Figure 3. Global IUS changes of two detergent emulsions at 30 °C and 45 °C
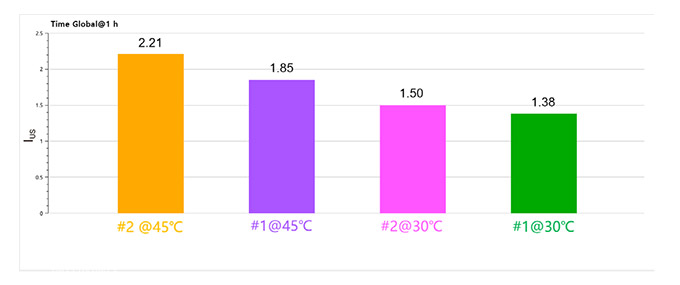
Figure 4. IUS of Two detergent emulsions @ 1 hour at 30 °C and 45 °C
Figures 2 and 3 show that the IUS values indicate good stability for the emulsified systems, attributed to the smaller particle sizes achieved through ultrasonic emulsification. The systems exhibited lower IUS values at 30 °C compared to 45 °C, suggesting better stability at lower temperatures.
Higher temperatures likely increase Brownian motion, leading to more frequent droplet collisions and coalescence, which reduces emulsion stability. Detergent #1 consistently had lower IUS values than detergent #2, indicating more stable emulsions.
The zeta potential and particle size analysis revealed that although both detergents produced emulsions with similar particle sizes, detergent #1 had smaller particles and a higher charge density, which enhanced emulsion stability.
Overall, detergent #1 demonstrated superior emulsion stability due to its finer particle size and higher charge. Additionally, lower temperatures were more effective for removing soybean oil with both detergents.
Conclusion
The BeScan Lab system offers rapid and precise assessments of emulsion stability, aiding in evaluating the cleaning effectiveness of detergents. Detergent #1 exhibits superior performance with a more stable emulsion, owing to forming smaller particle size and higher zeta potential. By streamlining the R&D process and ensuring consistent product quality, the BeScan Lab system effectively supports the development of high-performance detergent formulations.
About the Authors
 |
Xin Yan Application Engineer @ Bettersize Instruments |
 |
Dr. Hui Ning Chief Product Officer @ Bettersize Instruments |
|
BeScan Lab Stability Analyzer
|
 |
Rate this article
