What is zeta potential?
2023-05-25WIKI
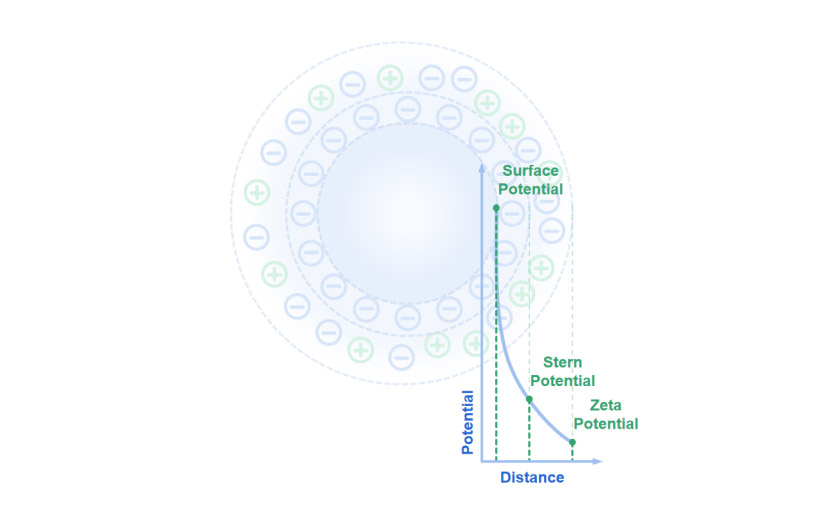
Particles carry charges on their surfaces in aqueous systems, surrounded by counter-ions that form a firm inner layer called the Stern plane and an outer shear plane. The electrostatic potential reaches the maximum at the particle surface, which is called the surface potential. The Stern plane is located at the electrical double layer's inner region, where ions are strongly bonded. The electrostatic potential at the Stern plane is called the Stern potential.
The shear plane describes the circle where the number of ions, both positive and negative, are equal, and thus an electrochemical equilibrium is established at the particle-liquid interface. The electrostatic potential in this region is called zeta potential. The zeta potential can be regarded as the potential difference between the dispersion medium and the stationary layer of the fluid attached to the particle layer.
A colloidal system with a higher zeta potential tends to be more stable and less likely to form aggregates, while lower zeta potentials lead to flocculation and coagulation of the particles due to Van der Waals forces. Measurement of zeta potential is an advantageous method to evaluate the stability of the colloidal system, which is widely applied in many fields, including ceramics, paints, inks, pharmaceuticals, water treatment, food, and beverage emulsions.